Switzerland-based CRISPR Therapeutics has quietly been revolutionizing the biomedical industry for over a decade. Recently, these advancements have crystallized into concrete results, as CRISPR’s gene therapy has recently been approved for human use. The therapy helps cure sickle cell disease (SCD) and beta-thalassemia using gene editing, based on the bacterial immune system. It has only been approved in the United Kingdom thus far, but worldwide implementations are expected soon.
The entire therapy was named Casgevy and was developed through a joint effort by Vertex Pharmaceuticals and CRISPR Therapeutics. It was approved recently by the UK’s Medicine and Healthcare Products Regulatory Agency following a large delay caused by the lack of resources after Brexit. Jeremy Hunt, the UK chancellor, dedicated 10 million pounds from the spring Budget to the agency to put in place the “quickest and simplest regulatory approval in the world for companies seeking rapid market access.” CRISPR capitalized on this endorsement and obtained the license needed to start administering the therapy. In just eleven years, it has evolved from the initial discovery to the patient-ready drug, and this staggering process earned Jennifer Doudna and Emmanuelle Charpentier – the scientists responsible for the therapy – a Nobel Prize in 2020. According to Doudna’s comment to the Financial Times, CRISPR’s rapid implementation caught the researchers off guard, and she estimates that tens of billions of dollars in value along with “many, many thousands” of jobs have been created as a result.
SCD and beta-thalassemia are life-long diseases caused by mutations in hemoglobin-producing genes, which effectively enable red blood cells to transport oxygen throughout the body. People with SCD often develop anemia as a consequence of its effect on cells that end up blocking blood vessels once. Similarly, beta-thalassemia leads to severe anemia due to its restrictions on hemoglobin production. Both can cause “pain crises” in which the individual experiences sudden and sharp pain related to improper blood flow. Around 20 million people struggle with SCD worldwide, including 100,000 in the USA and 15,000 in the UK. On the other hand, beta-thalassemia affects 1 in 100,000 people worldwide. Evidently, the relatively small number of people with these two compared to more common diseases has been the reason for the slow development in the research for a cure.
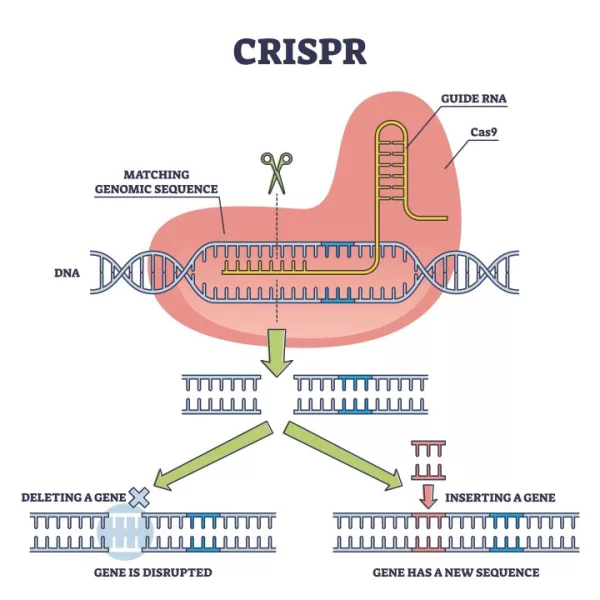
Casgevy works by cutting genes out of DNA using an enzyme called Cas9. It targets BCL11A, a gene responsible for producing a protein that switches the fetal version of hemoglobin to the adult one. The aim is to disable this gene since the adult proteins are defective in an individual with SCD or beta-thalassemia. In order to achieve this, stem cells are extracted from the patient’s bone marrow and the gene is edited using Casgevy in the lab. Following this process, the patients must spend at least a month in the hospital to facilitate red blood cell production. Up until now, bone marrow transplants have been the only possible remedy for such diseases and those run the risk of rejection, an issue significantly less prominent in this case.
The therapy’s administration has seen success so far, with promising results after the first batch of tests conducted. Twenty-eight out of 29 patients with SCD and beta-thalassemia experienced no severe pain crises for at least a year after the fact. Additionally, 39 out of 42 patients with beta-thalassemia did not need red blood cell transfusions after the treatment. Such results fill the researchers with optimism as the drug obtains approval to be administered universally but not without some reservations. There are some concerns regarding undesired side effects of Casgevy, namely unintended changes to other parts of the genome. This has not significantly deterred the drug’s potency, but the risk still remains present.
By December, the FDA should rule on approving the drug for treatment in the USA. Other countries are expected to follow suit, such as the EU’s European Medicines Agency and the Saudi Food and Drug Authority. However, its legal status will not be enough to ensure everyone’s access to the therapy as it is expected to launch with an extremely high cost associated. Currently, it is estimated that Casgevy will cost upwards of 1 million pounds in the UK, while the US price is projected at over $2 million per person. According to Reuters, the treatment would only be cost-effective at 1.5 million pounds or $1.9 million.

As expected, CRISPR Therapeutics stock (CRSP) has seen a massive hike recently, going against the industry as a whole. Over the course of the last three months, CRSP has seen a 41.9% increase, compared to the Medical-Biomed/Genetics market return of -11.2%. The stock could potentially rise to substantially higher levels following FDA approval and the therapy’s implementation in the European Union and Saudi Arabia as well.
Although there seems to be a long way to go to optimize this drug and ensure its access to everyone, the advancements made by CRISPR can only be characterized as a great step in the right direction.
Cover Image Source: https://www.iberdrola.com/innovation/genetic-modification-crispr.







